The Medidata RAVE Training Manual is a comprehensive guide designed to help users master the Medidata RAVE EDC system for clinical trial management. It provides a step-by-step approach to understanding key features, navigation, and data entry processes, ensuring efficient and accurate trial execution.
Overview of Medidata RAVE EDC System
Medidata RAVE is a powerful Electronic Data Capture (EDC) system designed to streamline clinical trial data collection and management. It offers a user-friendly interface for capturing, managing, and analyzing data in real-time. The system supports scalable solutions for trials of all sizes, ensuring data accuracy and compliance with regulatory standards. RAVE enables seamless collaboration among stakeholders, from investigators to sponsors, by providing role-based access and customizable workflows. Its advanced features, such as data validation and reporting tools, enhance efficiency and decision-making. As a widely adopted platform in the clinical research industry, Medidata RAVE is essential for modern clinical trial management.
Purpose of the Training Manual
The Medidata RAVE Training Manual is designed to equip users with the knowledge and skills needed to effectively utilize the RAVE EDC system. Its primary purpose is to provide a clear, structured approach to learning the platform, ensuring users can navigate, manage, and analyze clinical trial data efficiently. The manual is tailored for both new users and Read Only users, offering a comprehensive understanding of RAVE’s core functionalities. By following the manual, users gain the ability to perform essential tasks, such as data entry, report generation, and study management. It serves as a foundational resource for mastering RAVE, enabling users to contribute effectively to clinical trial success.
Target Audience for the Manual
The Medidata RAVE Training Manual is primarily designed for new users of the RAVE EDC system, including Read Only users who need to access and understand clinical trial data. It is also beneficial for clinical trial managers, data managers, and research coordinators involved in studies utilizing the RAVE platform. The manual is tailored to ensure that all users, regardless of their role, can gain a comprehensive understanding of the system’s features and functionalities. Additionally, it serves as a valuable resource for trainers and study team members who require a structured approach to learning and implementing RAVE in their daily operations. The manual’s content is generalized to accommodate a wide range of users participating in clinical trials.
Course Description and Objectives
The course provides a comprehensive guide for new users and Read Only users, covering access, navigation, and essential tasks in the RAVE EDC system.
Course Overview for New Users
The course is designed as a comprehensive guide for new users, providing a detailed introduction to the Medidata RAVE EDC system. It covers the basics of RAVE, including navigation, data entry, and reporting. The training is structured to ensure users gain hands-on experience with the platform, focusing on essential tasks and workflows. By completing the course, participants will understand how to access and utilize key features, ensuring efficient and accurate data management. The course also includes practical exercises and real-world examples to reinforce learning. This foundational training is crucial for all new users to master the core functionalities of RAVE EDC, enabling them to contribute effectively to clinical trial management.
Key Objectives of the Training
The primary objectives of the Medidata RAVE training are to familiarize users with the RAVE EDC system and ensure they can master its core functionalities. Participants will learn how to navigate the platform, perform data entry, and understand reporting features. The training also aims to enhance understanding of data management processes and improve efficiency in clinical trial execution. By the end of the course, users will be able to confidently perform essential tasks and apply best practices for data accuracy and compliance. The training emphasizes hands-on exercises and real-world examples to reinforce learning, ensuring participants are well-prepared to contribute effectively to clinical trial management.
Expected Outcomes for Participants
Upon completing the Medidata RAVE training, participants will gain proficiency in using the RAVE EDC system for clinical trial management. They will be able to confidently navigate the platform, perform accurate data entry, and generate meaningful reports. Participants will also understand how to manage user roles and permissions effectively and apply best practices for data validation. The training ensures that users can streamline workflows and enhance productivity in their daily tasks. By mastering these skills, participants will be well-equipped to contribute effectively to clinical trials and ensure high-quality data management. The training also prepares users to leverage advanced features of RAVE EDC for customized study solutions.
Rave Basics Training
Rave Basics Training provides a foundational understanding of the Medidata RAVE EDC system, covering key features and essential functionalities for all users to perform tasks efficiently.
The Rave Basics Training serves as the foundation for understanding the Medidata RAVE EDC system, designed for new users and Read Only users. It covers essential tasks such as data entry, navigation, and key features, ensuring a smooth transition into the system. This training provides a hands-on approach with practical examples to build proficiency in using RAVE for clinical trial management. By focusing on core functionalities, participants gain the confidence to perform daily tasks efficiently. The Rave Basics Training is a critical first step for anyone looking to master the Medidata RAVE EDC system, laying the groundwork for more advanced training in the future.
Key Features of Rave EDC
Medidata RAVE EDC is a robust electronic data capture system designed to streamline clinical trial management. Its key features include real-time data entry, advanced reporting, and data validation to ensure accuracy. The system offers customizable workflows and role-based permissions, allowing tailored access for different user roles. RAVE also supports integration with external systems and provides comprehensive audit trails for compliance. Its user-friendly interface and scalable architecture make it suitable for trials of all sizes. These features collectively enhance efficiency, accuracy, and compliance in clinical trial data management, making RAVE a powerful tool for researchers and study teams worldwide.
Importance of Rave Basics Training
Rave Basics Training is essential for users to gain a foundational understanding of the Medidata RAVE EDC system. It equips users with the core skills needed to navigate the platform, perform data entry, and understand data validation. This training ensures that users can efficiently manage clinical trial data while maintaining accuracy and compliance. By mastering the basics, users can confidently perform daily tasks and collaborate effectively with team members. The training also serves as a stepping stone for advanced features, making it a critical starting point for all new users. Investing time in Rave Basics ensures long-term proficiency and successful trial outcomes.

Navigating the Rave EDC System
Navigating Rave EDC involves mastering main menus, understanding user roles, and utilizing browsing tips to efficiently access and manage clinical trial data and tasks within the platform.
Main Menus and Functions
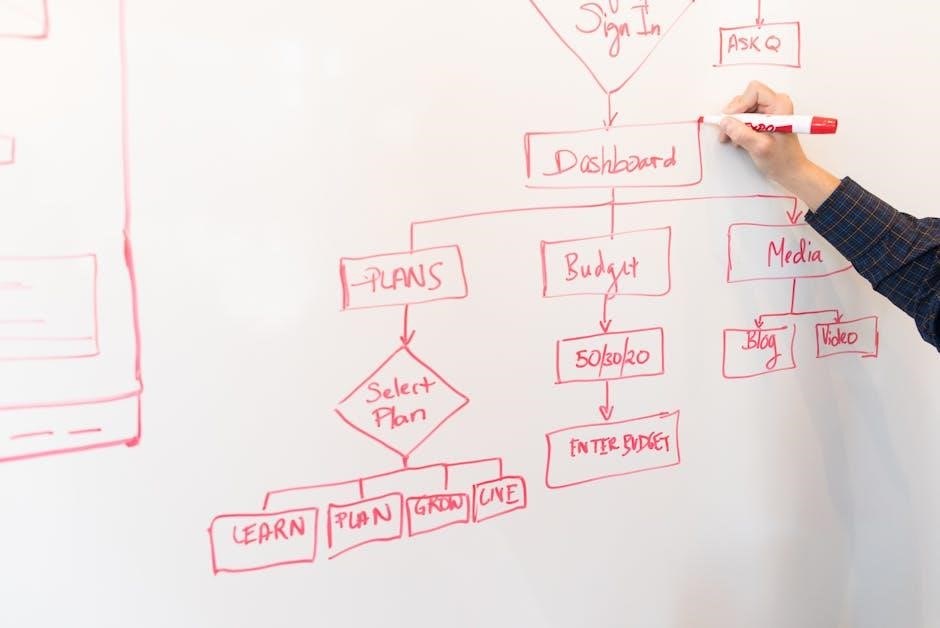
The main menus in Medidata RAVE serve as the central hub for accessing key features and functions. These menus are designed to streamline navigation, allowing users to efficiently manage clinical trial data and tasks. The primary menus include options for Study Browser, Tasks, and Reports, each providing direct access to essential tools. For instance, the Tasks pane enables users to view and complete assigned activities, while the Study Browser offers a detailed overview of study progress. Understanding these menus is crucial for effective navigation, as they simplify access to critical functionalities like data entry, reporting, and user management. Proper utilization of these features ensures a seamless experience in managing clinical trials within the Rave EDC system.
Understanding User Roles
Understanding user roles is essential for effective use of the Medidata RAVE EDC system. Each role defines the level of access and permissions within the platform, ensuring proper segregation of duties. Common roles include Study Administrator, Data Manager, and Read-Only User, each with distinct responsibilities. For instance, a Study Administrator can configure study settings and manage user permissions, while a Data Manager focuses on data entry and validation. Read-Only Users can view data but cannot make changes. Assigning roles appropriately ensures system security and compliance with clinical trial standards. Familiarizing oneself with these roles is crucial for efficient collaboration and maintaining data integrity in the RAVE EDC environment.
Browsing and Navigation Tips
Browsing and navigating the Medidata RAVE EDC system efficiently requires a clear understanding of its layout and functionality. Start by familiarizing yourself with the main menus and their associated functions, as they provide access to key features like data entry, reporting, and user management. Utilize the Tasks pane to quickly access frequently used tools and eLearnings. To enhance navigation, use the breadcrumb trail at the top of each page to track your path and easily return to previous sections. Additionally, consider customizing your view by adding favorites for commonly used pages. By mastering these navigation tips, you can streamline your workflow and reduce the time spent searching for features, ensuring a more efficient and productive experience in RAVE EDC.
User Roles and Permissions
Understanding user roles and permissions is crucial for effective use of the Medidata RAVE EDC system. This section explains how roles are defined and permissions are assigned to ensure secure and efficient collaboration in clinical trial management.
Types of User Roles in Rave
In the Medidata RAVE EDC system, user roles are defined to ensure proper access and functionality. Common roles include Read Only, Data Entry, Study Manager, and Administrator. Each role has specific permissions, such as viewing data, entering information, managing study settings, or configuring system-wide options. These roles are designed to align with the responsibilities of different team members in clinical trials, ensuring that users only access features relevant to their tasks. Understanding these roles is essential for maintaining data integrity and collaboration efficiency. The manual provides detailed descriptions of each role to help organizations assign permissions effectively and streamline workflow. Proper role assignment ensures compliance with security and operational standards in clinical trial management.
Understanding Permissions

Permissions in Medidata RAVE are critical for ensuring that users can only access and modify data according to their assigned roles. These permissions are tied to specific user roles and are designed to maintain data integrity and security. For example, a Data Entry user may only edit certain fields, while an Administrator has full access to configure the system. Understanding permissions is essential for preventing unauthorized changes and ensuring compliance with clinical trial standards. The training manual provides detailed explanations of how permissions are structured and how they align with user roles. This knowledge helps users navigate the system confidently while adhering to security protocols. Proper permission management is a cornerstone of effective RAVE EDC system utilization.
Best Practices for Role Management
Effective role management in Medidata RAVE is essential for maintaining data integrity and system security. Define roles clearly based on user responsibilities and ensure permissions align with job functions. Regularly review and update roles to reflect changes in staff or study requirements. Document role changes and communicate updates to all relevant users. Provide training to help users understand their permissions and responsibilities. Use built-in tools to monitor access and audit trails for compliance. Avoid over-privileging users to minimize risks of unauthorized data modifications. By following these best practices, organizations can ensure efficient and secure use of the RAVE EDC system while maintaining compliance with clinical trial standards.

Data Entry and Management
The Medidata RAVE EDC system streamlines clinical trial data entry with efficient tools for accurate and timely input. It ensures data validation and quality control, while best practices guide users in managing data effectively.
Data Entry Process in Rave

The data entry process in Medidata RAVE is designed to ensure accuracy and efficiency in clinical trial management. Users can access the system through a secure login and navigate to the relevant study and patient data. The platform provides intuitive forms for entering data, with real-time validation to minimize errors. Data is organized by study, site, and patient, making it easy to locate and update information. User roles determine access levels, ensuring that only authorized personnel can modify data. The system also supports batch entry for efficient data management. By following best practices, users can maintain data integrity and ensure compliance with regulatory standards. This process is critical for successful clinical trial outcomes.
Data Validation and Quality Control
Data validation and quality control are critical components of the Medidata RAVE EDC system, ensuring the accuracy and reliability of clinical trial data. The system employs real-time validation checks to identify and flag discrepancies, preventing errors during data entry. Users can address issues through discrepancy management tools, which allow for corrections and explanations. Quality control measures include audit trails and electronic signatures, providing a transparent record of all data modifications. These features help maintain data integrity and ensure compliance with regulatory requirements. By leveraging these tools, users can confidently manage high-quality data, which is essential for the success of clinical trials and subsequent analyses.
Best Practices for Data Management
Effective data management in Medidata RAVE requires adherence to best practices to ensure accuracy, consistency, and compliance. Start by standardizing data entry processes across all users to minimize errors. Regularly review and audit data to identify discrepancies early, leveraging RAVE’s validation tools. Assign clear user roles and permissions to maintain data security and accountability. Document all data management procedures and ensure proper training for users. Additionally, utilize RAVE’s reporting features to monitor data quality and progress. By following these practices, users can optimize data integrity, streamline workflows, and ensure successful clinical trial outcomes. Consistent adherence to these guidelines will enhance overall efficiency and reliability in data management within the RAVE EDC system.
Reporting in Rave EDC
RAVE EDC offers robust reporting tools to generate, customize, and schedule reports, enabling efficient monitoring and analysis of clinical trial data. Users can easily export reports for further review and decision-making.
Types of Reports in Rave
RAVE EDC provides various types of reports to support clinical trial management, including study progress reports, data status reports, and adverse event reports. These reports offer insights into trial performance, data accuracy, and patient safety. Additionally, RAVE generates patient profile reports and site performance reports, enabling detailed monitoring and analysis. Users can also create custom reports tailored to specific study needs, ensuring flexibility and efficiency in data review. These reporting options empower stakeholders to make informed decisions and maintain compliance with regulatory requirements.
Customizing Reports
Customizing reports in RAVE EDC allows users to tailor outputs to specific study requirements, enhancing data analysis and decision-making. Users can modify report layouts, apply filters, and select relevant data fields to focus on key metrics. Advanced features enable the inclusion of custom calculations and visualizations, providing deeper insights. Additionally, reports can be scheduled for automatic generation and exported in various formats, such as PDF or Excel, for easy sharing and further analysis. This flexibility ensures that stakeholders receive precise and actionable data, supporting efficient trial management and regulatory compliance. Customization options empower users to align reports with study goals, improving overall trial efficiency and outcomes.
Scheduling and Exporting Reports
Scheduling and exporting reports in RAVE EDC streamlines data accessibility and sharing. Users can schedule reports to generate automatically at specific intervals, ensuring timely updates without manual intervention. Export options allow data to be saved in formats like PDF, Excel, or CSV, facilitating further analysis and reporting. Scheduled reports can be configured to meet specific study needs, reducing manual effort and enhancing efficiency. Additionally, exported reports can be easily shared with stakeholders, supporting collaboration and decision-making. Best practices include setting up notifications for report completion and organizing exported files for easy access. This feature ensures that critical data is readily available, supporting seamless trial management and compliance with regulatory requirements.

Advanced Features of Rave EDC
Advanced features in Rave EDC unlock efficiency and innovation in clinical trial management. Tools like advanced analytics, custom workflows, and study-specific configurations empower users to streamline processes and enhance productivity.
The advanced features of Medidata RAVE EDC are designed to enhance clinical trial management through innovative tools and functionalities. These features include advanced data analytics, custom workflows, and study-specific configurations, enabling users to streamline complex processes. By leveraging these tools, users can improve data accuracy, accelerate trial execution, and enhance decision-making. The Medidata RAVE Training Manual provides detailed guidance on how to utilize these advanced features effectively, ensuring users can maximize the system’s capabilities. Whether it’s customizing reports or integrating advanced analytics, the manual offers a comprehensive roadmap for mastering RAVE’s advanced functionalities, making it an essential resource for experienced users aiming to optimize their clinical trial operations.
Advanced Data Analytics
Advanced data analytics in Medidata RAVE EDC empower users to transform raw data into actionable insights, enhancing clinical trial decision-making. The system offers tools for customizable reporting, enabling users to create tailored dashboards and visualizations. Real-time data monitoring and analysis capabilities allow for proactive identification of trends and anomalies, ensuring data integrity and compliance. These features are particularly valuable for complex studies, where granular data analysis is critical. The Medidata RAVE Training Manual provides detailed guidance on leveraging these advanced analytics tools, helping users to maximize efficiency and accuracy in their clinical trial operations. By mastering these capabilities, users can make data-driven decisions with confidence, ultimately supporting the success of their trials.
Customizing Rave for Specific Studies

Customizing Medidata RAVE for specific studies ensures tailored solutions to meet unique research requirements. The system allows users to configure study-specific settings, such as custom fields, workflows, and validation rules, ensuring data collection aligns with trial objectives. Advanced features enable the creation of study-specific forms and adapters to integrate with external systems, enhancing operational efficiency. The Medidata RAVE Training Manual provides detailed guidance on how to implement these customizations effectively. By leveraging these tools, users can streamline data management processes, reduce errors, and ensure compliance with regulatory standards. Customization options empower sponsors and researchers to adapt RAVE to their specific needs, making it a versatile and powerful tool for clinical trial management.

Conclusion and Next Steps

In conclusion, the Medidata RAVE Training Manual provides a thorough understanding of the EDC system. Users are now equipped to efficiently manage clinical trials. For further learning, explore additional resources or contact Medidata support for assistance.
Summary of Key Takeaways
The Medidata RAVE Training Manual provides a comprehensive understanding of the EDC system, focusing on data management, navigation, and advanced features. Users gain hands-on knowledge of data entry, validation, and reporting, ensuring accuracy and efficiency in clinical trials. The manual emphasizes best practices for user roles, permissions, and study customization. Participants are equipped to master RAVE basics and leverage its tools for streamlined trial execution. This guide is essential for new and experienced users, offering a clear path to proficiency in RAVE EDC. For further growth, users are encouraged to explore additional resources and eLearnings to deepen their expertise.
Additional Resources for Further Learning
For continued growth, users can access eLearnings through the RAVE EDC system’s Tasks pane, offering in-depth training modules. These resources cover advanced features like data analytics and customizations. Additionally, the Medidata RAVE Training Manual PDF provides a step-by-step guide for mastering the platform. Users can also explore online forums and community support for troubleshooting and best practices. Regular webinars and updates from Medidata ensure users stay informed about new functionalities. By leveraging these resources, users can enhance their proficiency and optimize their use of the RAVE EDC system for efficient clinical trial management.